For a full bibliography please visit PubMed
Selected Publications
An IL-4 signalling axis in bone marrow drives pro-tumorigenic myelopoiesis.
Nelson M LaMarche, Samarth Hegde, Matthew D Park, Barbara B Maier, (…), Brian S Kim, Thomas U Marron, Miriam Merad. (2024) Nature
-
Myeloid cells are known to suppress antitumour immunity. However, the molecular drivers of immunosuppressive myeloid cell states are not well defined. Here we used single-cell RNA sequencing of human and mouse non-small cell lung cancer (NSCLC) lesions, and found that in both species the type 2 cytokine interleukin-4 (IL-4) was predicted to be the primary driver of the tumour-infiltrating monocyte-derived macrophage phenotype. Using a panel of conditional knockout mice, we found that only deletion of the IL-4 receptor IL-4Rα in early myeloid progenitors in bone marrow reduced tumour burden, whereas deletion of IL-4Rα in downstream mature myeloid cells had no effect. Mechanistically, IL-4 derived from bone marrow basophils and eosinophils acted on granulocyte-monocyte progenitors to transcriptionally programme the development of immunosuppressive tumour-promoting myeloid cells. Consequentially, depletion of basophils profoundly reduced tumour burden and normalized myelopoiesis. We subsequently initiated a clinical trial of the IL-4Rα blocking antibody dupilumab 2-5 given in conjunction with PD-1/PD-L1 checkpoint blockade in patients with relapsed or refractory NSCLC who had progressed on PD-1/PD-L1 blockade alone (ClinicalTrials.gov identifier NCT05013450). Dupilumab supplementation reduced circulating monocytes, expanded tumour-infiltrating CD8 T cells, and in one out of six patients, drove a near-complete clinical response two months after treatment. Our study defines a central role for IL-4 in controlling immunosuppressive myelopoiesis in cancer, identifies a novel combination therapy for immune checkpoint blockade in humans, and highlights cancer as a systemic malady that requires therapeutic strategies beyond the primary disease site.
Circulating senescent myeloid cells infiltrate the brain and cause neurodegeneration in histiocytic disorders.
C Matthias Wilk, Flurin Cathomas, Orsolya Török, Jessica Le Berichel, Matthew D Park, (…), Brian S Kim, Thomas U Marron, Miriam Merad. (2023) Immunity
-
Neurodegenerative diseases (ND) are characterized by progressive loss of neuronal function. Mechanisms of ND pathogenesis are incompletely understood, hampering the development of effective therapies. Langerhans cell histiocytosis (LCH) is an inflammatory neoplastic disorder caused by hematopoietic progenitors expressing mitogen-activated protein kinase (MAPK)-activating mutations that differentiate into senescent myeloid cells that drive lesion formation. Some individuals with LCH subsequently develop progressive and incurable neurodegeneration (LCH-ND). Here, we showed that LCH-ND was caused by myeloid cells that were clonal with peripheral LCH cells. Circulating BRAFV600E+ myeloid cells caused the breakdown of the blood-brain barrier (BBB), enhancing migration into the brain parenchyma where they differentiated into senescent, inflammatory CD11a+ macrophages that accumulated in the brainstem and cerebellum. Blocking MAPK activity and senescence programs reduced peripheral inflammation, brain parenchymal infiltration, neuroinflammation, neuronal damage and improved neurological outcome in preclinical LCH-ND. MAPK activation and senescence programs in circulating myeloid cells represent targetable mechanisms of LCH-ND.
Intratumoral dendritic cell-CD4+ T helper cell niches enable CD8+ T cell differentiation following PD-1 blockade in hepatocellular carcinoma.
Assaf Magen, Pauline Hamon, Nathalie Fiaschi, Brian Y Soong, (…), Gavin Thurston, Alice O Kamphorst, Miriam Merad. (2023) Nature Medicine
-
Despite no apparent defects in T cell priming and recruitment to tumors, a large subset of T cell rich tumors fail to respond to immune checkpoint blockade (ICB). We leveraged a neoadjuvant anti-PD-1 trial in patients with hepatocellular carcinoma (HCC), as well as additional samples collected from patients treated off-label, to explore correlates of response to ICB within T cell-rich tumors. We show that ICB response correlated with the clonal expansion of intratumoral CXCL13+CH25H+IL-21+PD-1+CD4+ T helper cells ("CXCL13+ TH") and Granzyme K+ PD-1+ effector-like CD8+ T cells, whereas terminally exhausted CD39hiTOXhiPD-1hiCD8+ T cells dominated in nonresponders. CD4+ and CD8+ T cell clones that expanded post-treatment were found in pretreatment biopsies. Notably, PD-1+TCF-1+ (Progenitor-exhausted) CD8+ T cells shared clones mainly with effector-like cells in responders or terminally exhausted cells in nonresponders, suggesting that local CD8+ T cell differentiation occurs upon ICB. We found that these Progenitor CD8+ T cells interact with CXCL13+ TH within cellular triads around dendritic cells enriched in maturation and regulatory molecules, or "mregDC". These results suggest that discrete intratumoral niches that include mregDC and CXCL13+ TH control the differentiation of tumor-specific Progenitor exhasuted CD8+ T cells following ICB.
TREM2 macrophages drive NK cell paucity and dysfunction in lung cancer
Matthew D Park, Ivan Reyes-Torres, Jessica LeBerichel, Pauline Hamon, (…), Marco Colonna, Thomas U Marron, Miriam Merad. (2023) Nature Immunologie
-
Natural killer (NK) cells are commonly reduced in human tumors, enabling many to evade surveillance. Here, we sought to identify cues that alter NK cell activity in tumors. We found that, in human lung cancer, the presence of NK cells inversely correlated with that of monocyte-derived macrophages (mo-macs). In a murine model of lung adenocarcinoma, we show that engulfment of tumor debris by mo-macs triggers a pro-tumorigenic program governed by triggering receptor expressed on myeloid cells 2 (TREM2). Genetic deletion of Trem2 rescued NK cell accumulation and enabled an NK cell-mediated regression of lung tumors. TREM2+ mo-macs reduced NK cell activity by modulating interleukin (IL)-18/IL-18BP decoy interactions and IL-15 production. Notably, TREM2 blockade synergized with an NK cell-activating agent to further inhibit tumor growth. Altogether, our findings identify a new axis, in which TREM2+ mo-macs suppress NK cell accumulation and cytolytic activity. Dual targeting of macrophages and NK cells represents a new strategy to boost antitumor immunity.
A shift in lung macrophage composition is associated with COVID-19 severity and recovery.
Steven T Chen, Matthew D Park, Diane Marie Del Valle, (…), Ephraim Kenigsberg, Alexander W Charney, Miriam Merad. (2022) Science Translational Medicine
-
Although it has been more than 2 years since the start of the coronavirus disease 2019 (COVID-19) pandemic, COVID-19 continues to be a worldwide health crisis. Despite the development of preventive vaccines, therapies to treat COVID-19 and other inflammatory diseases remain a major unmet need in medicine. Our study sought to identify drivers of disease severity and mortality to develop tailored immunotherapy strategies to halt disease progression. We assembled the Mount Sinai COVID-19 Biobank, which was composed of almost 600 hospitalized patients followed longitudinally through the peak of the pandemic in 2020. Moderate disease and survival were associated with a stronger antigen presentation and effector T cell signature. In contrast, severe disease and death were associated with an altered antigen presentation signature, increased numbers of inflammatory immature myeloid cells, and extrafollicular activated B cells that have been previously associated with autoantibody formation. In severely ill patients with COVID-19, lung tissue-resident alveolar macrophages not only were drastically depleted but also had an altered antigen presentation signature, which coincided with an influx of inflammatory monocytes and monocyte-derived macrophages. In addition, we found that the size of the alveolar macrophage pool correlated with patient outcome and that alveolar macrophage numbers and functionality were restored to homeostasis in patients who recovered from COVID-19. These data suggest that local and systemic myeloid cell dysregulation are drivers of COVID-19 severity and modulation of alveolar macrophage numbers and activity in the lung may be a viable therapeutic strategy for the treatment of critical inflammatory lung diseases.
Single-cell analysis of human non-small cell lung cancer lesions refines tumor classification and patient stratification.
Andrew M. Leader, John A. Grout, Barbara B. Maier, (…), Thomas U. Marron, Ephraim Kenigsberg, Miriam Merad. (2021) Cancer Cell.
-
Immunotherapy is a mainstay of non-small cell lung cancer (NSCLC) management. While tumor mutational burden (TMB) correlates with response to immunotherapy, little is known about the relationship between the baseline immune response and tumor genotype. Using single-cell RNA sequencing, we profiled 361,929 cells from 35 early-stage NSCLC lesions. We identified a cellular module consisting of PDCD1+CXCL13+ activated T cells, IgG+ plasma cells, and SPP1+ macrophages, referred to as the lung cancer activation module (LCAMhi). We confirmed LCAMhi enrichment in multiple NSCLC cohorts, and paired CITE-seq established an antibody panel to identify LCAMhi lesions. LCAM presence was found to be independent of overall immune cell content and correlated with TMB, cancer testis antigens, and TP53 mutations. High baseline LCAM scores correlated with enhanced NSCLC response to immunotherapy even in patients with above median TMB, suggesting that immune cell composition, while correlated with TMB, may be a nonredundant biomarker of response to immunotherapy. text goes here
Tissue-resident macrophages provide a pro-tumorigenic niche to early NSCLC cells.
María Casanova-Acebes, Erica Dalla, Andrew M Leader, Jessica LeBerichel, Jovan Nikolic, (…), Philippe Benaroch, Julio A Aguirre-Ghiso, Miriam Merad. (2021) Nature.
-
Macrophages have a key role in shaping the tumour microenvironment (TME), tumour immunity and response to immunotherapy, which makes them an important target for cancer treatment1,2. However, modulating macrophages has proved extremely difficult, as we still lack a complete understanding of the molecular and functional diversity of the tumour macrophage compartment. Macrophages arise from two distinct lineages. Tissue-resident macrophages self-renew locally, independent of adult haematopoiesis3-5, whereas short-lived monocyte-derived macrophages arise from adult haematopoietic stem cells, and accumulate mostly in inflamed lesions1. How these macrophage lineages contribute to the TME and cancer progression remains unclear. To explore the diversity of the macrophage compartment in human non-small cell lung carcinoma (NSCLC) lesions, here we performed single-cell RNA sequencing of tumour-associated leukocytes. We identified distinct populations of macrophages that were enriched in human and mouse lung tumours. Using lineage tracing, we discovered that these macrophage populations differ in origin and have a distinct temporal and spatial distribution in the TME. Tissue-resident macrophages accumulate close to tumour cells early during tumour formation to promote epithelial-mesenchymal transition and invasiveness in tumour cells, and they also induce a potent regulatory T cell response that protects tumour cells from adaptive immunity. Depletion of tissue-resident macrophages reduced the numbers and altered the phenotype of regulatory T cells, promoted the accumulation of CD8+ T cells and reduced tumour invasiveness and growth. During tumour growth, tissue-resident macrophages became redistributed at the periphery of the TME, which becomes dominated by monocyte-derived macrophages in both mouse and human NSCLC. This study identifies the contribution of tissue-resident macrophages to early lung cancer and establishes them as a target for the prevention and treatment of early lung cancer lesions.
BRAFV600E-induced senescence drives Langerhans cell histiocytosis pathophysiology.
Camille Bigenwald, Jessica Le Berichel, C Matthias Wilk, Rikhia Chakraborty, (…), Jennifer Picarsic, Carl E Allen, Miriam Merad. (2021) Nature Medicine.
-
Langerhans cell histiocytosis (LCH) is a potentially fatal condition characterized by granulomatous lesions with characteristic clonal mononuclear phagocytes (MNPs) harboring activating somatic mutations in mitogen-activated protein kinase (MAPK) pathway genes, most notably BRAFV600E. We recently discovered that the BRAFV600E mutation can also affect multipotent hematopoietic progenitor cells (HPCs) in multisystem LCH disease. How the BRAFV600E mutation in HPCs leads to LCH is not known. Here we show that enforced expression of the BRAFV600E mutation in early mouse and human multipotent HPCs induced a senescence program that led to HPC growth arrest, apoptosis resistance and a senescence-associated secretory phenotype (SASP). SASP, in turn, promoted HPC skewing toward the MNP lineage, leading to the accumulation of senescent MNPs in tissue and the formation of LCH lesions. Accordingly, elimination of senescent cells using INK-ATTAC transgenic mice, as well as pharmacologic blockade of SASP, improved LCH disease in mice. These results identify senescent cells as a new target for the treatment of LCH.
Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages.
Miriam Merad, Jerome C Martin. (2020) Nature Reviews Immunology.
-
The COVID-19 pandemic caused by infection with SARS-CoV-2 has led to more than 200,000 deaths worldwide. Several studies have now established that the hyperinflammatory response induced by SARS-CoV-2 is a major cause of disease severity and death in infected patients. Macrophages are a population of innate immune cells that sense and respond to microbial threats by producing inflammatory molecules that eliminate pathogens and promote tissue repair. However, a dysregulated macrophage response can be damaging to the host, as is seen in the macrophage activation syndrome induced by severe infections, including in infections with the related virus SARS-CoV. Here we describe the potentially pathological roles of macrophages during SARS-CoV-2 infection and discuss ongoing and prospective therapeutic strategies to modulate macrophage activation in patients with COVID-19.
A conserved dendritic-cell regulatory program limits antitumour immunity.
Barbara Maier, Andrew M Leader, Steven T Chen, Navpreet Tung, (…), Ephraim Kenigsberg, Brian D Brown, Miriam Merad. (2020) Nature.
-
Checkpoint blockade therapies have improved cancer treatment, but such immunotherapy regimens fail in a large subset of patients. Conventional type 1 dendritic cells (DC1s) control the response to checkpoint blockade in preclinical models and are associated with better overall survival in patients with cancer, reflecting the specialized ability of these cells to prime the responses of CD8+ T cells1-3. Paradoxically, however, DC1s can be found in tumours that resist checkpoint blockade, suggesting that the functions of these cells may be altered in some lesions. Here, using single-cell RNA sequencing in human and mouse non-small-cell lung cancers, we identify a cluster of dendritic cells (DCs) that we name 'mature DCs enriched in immunoregulatory molecules' (mregDCs), owing to their coexpression of immunoregulatory genes (Cd274, Pdcd1lg2 and Cd200) and maturation genes (Cd40, Ccr7 and Il12b). We find that the mregDC program is expressed by canonical DC1s and DC2s upon uptake of tumour antigens. We further find that upregulation of the programmed death ligand 1 protein-a key checkpoint molecule-in mregDCs is induced by the receptor tyrosine kinase AXL, while upregulation of interleukin (IL)-12 depends strictly on interferon-γ and is controlled negatively by IL-4 signalling. Blocking IL-4 enhances IL-12 production by tumour-antigen-bearing mregDC1s, expands the pool of tumour-infiltrating effector T cells and reduces tumour burden. We have therefore uncovered a regulatory module associated with tumour-antigen uptake that reduces DC1 functionality in human and mouse cancers.
RXRs control serous macrophage neonatal expansion and identity and contribute to ovarian cancer progression.
María Casanova-Acebes, María Piedad Menéndez-Gutiérrez, Jesús Porcuna, (…), Fátima Sánchez-Cabo, Miriam Merad, Mercedes Ricote. (2020) Nature Communications.
-
Tissue-resident macrophages (TRMs) populate all tissues and play key roles in homeostasis, immunity and repair. TRMs express a molecular program that is mostly shaped by tissue cues. However, TRM identity and the mechanisms that maintain TRMs in tissues remain poorly understood. We recently found that serous-cavity TRMs (LPMs) are highly enriched in RXR transcripts and RXR-response elements. Here, we show that RXRs control mouse serous-macrophage identity by regulating chromatin accessibility and the transcriptional regulation of canonical macrophage genes. RXR deficiency impairs neonatal expansion of the LPM pool and reduces the survival of adult LPMs through excess lipid accumulation. We also find that peritoneal LPMs infiltrate early ovarian tumours and that RXR deletion diminishes LPM accumulation in tumours and strongly reduces ovarian tumour progression in mice. Our study reveals that RXR signalling controls the maintenance of the serous macrophage pool and that targeting peritoneal LPMs may improve ovarian cancer outcomes.
Single-Cell Analysis of Crohn's Disease Lesions Identifies a Pathogenic Cellular Module Associated with Resistance to Anti-TNF Therapy.
Jerome C Martin, Christie Chang, Gilles Boschetti, (…), Miriam Merad, Judy H Cho, Ephraim Kenigsberg. (2019) Cell.
-
Clinical benefits of cytokine blockade in ileal Crohn's disease (iCD) are limited to a subset of patients. Here, we applied single-cell technologies to iCD lesions to address whether cellular heterogeneity contributes to treatment resistance. We found that a subset of patients expressed a unique cellular module in inflamed tissues that consisted of IgG plasma cells, inflammatory mononuclear phagocytes, activated T cells, and stromal cells, which we named the GIMATS module. Analysis of ligand-receptor interaction pairs identified a distinct network connectivity that likely drives the GIMATS module. Strikingly, the GIMATS module was also present in a subset of patients in four independent iCD cohorts (n = 441), and its presence at diagnosis correlated with failure to achieve durable corticosteroid-free remission upon anti-TNF therapy. These results emphasize the limitations of current diagnostic assays and the potential for single-cell mapping tools to identify novel biomarkers of treatment response and tailored therapeutic opportunities.
Dietary Intake Regulates the Circulating Inflammatory Monocyte Pool.
Stefan Jordan, Navpreet Tung, Maria Casanova-Acebes, (…), Marie-Luise Berres, Emily J Gallagher, Miriam Merad. (2019) Cell.
-
Caloric restriction is known to improve inflammatory and autoimmune diseases. However, the mechanisms by which reduced caloric intake modulates inflammation are poorly understood. Here we show that short-term fasting reduced monocyte metabolic and inflammatory activity and drastically reduced the number of circulating monocytes. Regulation of peripheral monocyte numbers was dependent on dietary glucose and protein levels. Specifically, we found that activation of the low-energy sensor 5'-AMP-activated protein kinase (AMPK) in hepatocytes and suppression of systemic CCL2 production by peroxisome proliferator-activator receptor alpha (PPARα) reduced monocyte mobilization from the bone marrow. Importantly, we show that fasting improves chronic inflammatory diseases without compromising monocyte emergency mobilization during acute infectious inflammation and tissue repair. These results reveal that caloric intake and liver energy sensors dictate the blood and tissue immune tone and link dietary habits to inflammatory disease outcome.
CSF-1 controls cerebellar microglia and is required for motor function and social interaction.
Veronika Kana, Fiona A Desland, Maria Casanova-Acebes, Pinar Ayata, (…), Hirofumi Morishita, Anne Schaefer, Miriam Merad. (2019) JEM.
-
Microglia, the brain resident macrophages, critically shape forebrain neuronal circuits. However, their precise function in the cerebellum is unknown. Here we show that human and mouse cerebellar microglia express a unique molecular program distinct from forebrain microglia. Cerebellar microglial identity was driven by the CSF-1R ligand CSF-1, independently of the alternate CSF-1R ligand, IL-34. Accordingly, CSF-1 depletion from Nestin+ cells led to severe depletion and transcriptional alterations of cerebellar microglia, while microglia in the forebrain remained intact. Strikingly, CSF-1 deficiency and alteration of cerebellar microglia were associated with reduced Purkinje cells, altered neuronal function, and defects in motor learning and social novelty interactions. These findings reveal a novel CSF-1-CSF-1R signaling-mediated mechanism that contributes to motor function and social behavior.
Host tissue determinants of tumour immunity.
Hélène Salmon, Romain Remark, Sacha Gnjatic, Miriam Merad (2019) Nature Reviews Cancer.
-
Although common evolutionary principles drive the growth of cancer cells regardless of the tissue of origin, the microenvironment in which tumours arise substantially differs across various organ sites. Recent studies have established that, in addition to cell-intrinsic effects, tumour growth regulation also depends on local cues driven by tissue environmental factors. In this Review, we discuss how tissue-specific determinants might influence tumour development and argue that unravelling the tissue-specific contribution to tumour immunity should help the development of precise immunotherapeutic strategies for patients with cancer.
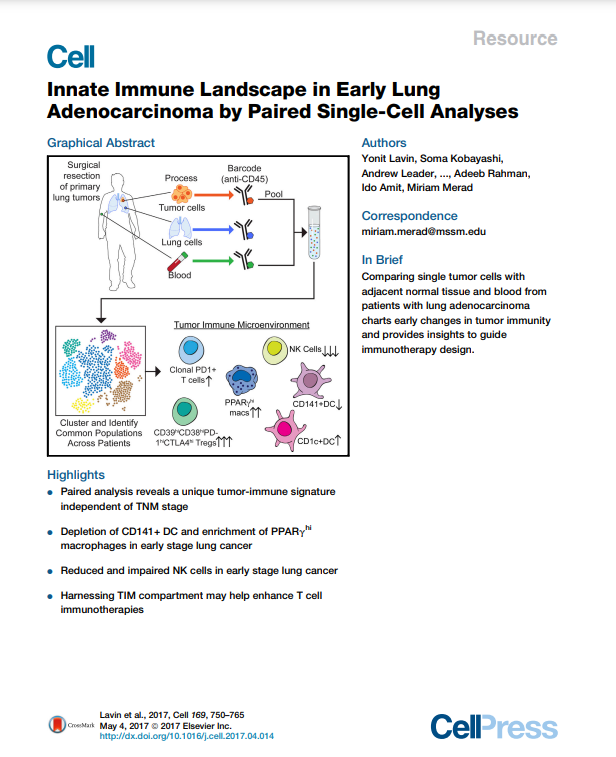
Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses.
Yonit Lavin, Soma Kobayashi, Andrew Leader, (…), Adeeb Rahman, Ido Amit, Miriam Merad. (2017) Cell.
-
To guide the design of immunotherapy strategies for patients with early stage lung tumors, we developed a multiscale immune profiling strategy to map the immune landscape of early lung adenocarcinoma lesions to search for tumor-driven immune changes. Utilizing a barcoding method that allows a simultaneous single-cell analysis of the tumor, non-involved lung, and blood cells, we provide a detailed immune cell atlas of early lung tumors. We show that stage I lung adenocarcinoma lesions already harbor significantly altered T cell and NK cell compartments. Moreover, we identified changes in tumor-infiltrating myeloid cell (TIM) subsets that likely compromise anti-tumor T cell immunity. Paired single-cell analyses thus offer valuable knowledge of tumor-driven immune changes, providing a powerful tool for the rational design of immune therapies.
Host-Protozoan Interactions Protect from Mucosal Infections through Activation of the Inflammasome.
Aleksey Chudnovskiy, Arthur Mortha, Veronika Kana, (…), Yasmine Belkaid, Michael E Grigg, Miriam Merad. (2016) Cell.
-
While conventional pathogenic protists have been extensively studied, there is an underappreciated constitutive protist microbiota that is an integral part of the vertebrate microbiome. The impact of these species on the host and their potential contributions to mucosal immune homeostasis remain poorly studied. Here, we show that the protozoan Tritrichomonas musculis activates the host epithelial inflammasome to induce IL-18 release. Epithelial-derived IL-18 promotes dendritic cell-driven Th1 and Th17 immunity and confers dramatic protection from mucosal bacterial infections. Along with its role as a "protistic" antibiotic, colonization with T. musculis exacerbates the development of T-cell-driven colitis and sporadic colorectal tumors. Our findings demonstrate a novel mutualistic host-protozoan interaction that increases mucosal host defenses at the cost of an increased risk of inflammatory disease.
Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition.
Hélène Salmon, Juliana Idoyaga, Adeeb Rahman, (…), Joshua Brody, Florent Ginhoux, Miriam Merad. (2016) Immunity.
-
Large numbers of melanoma lesions develop resistance to targeted inhibition of mutant BRAF or fail to respond to checkpoint blockade. We explored whether modulation of intratumoral antigen-presenting cells (APCs) could increase responses to these therapies. Using mouse melanoma models, we found that CD103(+) dendritic cells (DCs) were the only APCs transporting intact antigens to the lymph nodes and priming tumor-specific CD8(+) T cells. CD103(+) DCs were required to promote anti-tumoral effects upon blockade of the checkpoint ligand PD-L1; however, PD-L1 inhibition only led to partial responses. Systemic administration of the growth factor FLT3L followed by intratumoral poly I:C injections expanded and activated CD103(+) DC progenitors in the tumor, enhancing responses to BRAF and PD-L1 blockade and protecting mice from tumor rechallenge. Thus, the paucity of activated CD103(+) DCs in tumors limits checkpoint-blockade efficacy and combined FLT3L and poly I:C therapy can enhance tumor responses to checkpoint and BRAF blockade.
Regulation of macrophage development and function in peripheral tissues.
Yonit Lavin, Arthur Mortha, Adeeb Rahman, Miriam Merad (2015) Nature Reviews Immunology.
-
Large numbers of melanoma lesions develop resistance to targeted inhibition of mutant BRAF or fail to respond to checkpoint blockade. We explored whether modulation of intratumoral antigen-presenting cells (APCs) could increase responses to these therapies. Using mouse melanoma models, we found that CD103(+) dendritic cells (DCs) were the only APCs transporting intact antigens to the lymph nodes and priming tumor-specific CD8(+) T cells. CD103(+) DCs were required to promote anti-tumoral effects upon blockade of the checkpoint ligand PD-L1; however, PD-L1 inhibition only led to partial responses. Systemic administration of the growth factor FLT3L followed by intratumoral poly I:C injections expanded and activated CD103(+) DC progenitors in the tumor, enhancing responses to BRAF and PD-L1 blockade and protecting mice from tumor rechallenge. Thus, the paucity of activated CD103(+) DCs in tumors limits checkpoint-blockade efficacy and combined FLT3L and poly I:C therapy can enhance tumor responses to checkpoint and BRAF blockade.
CDKN1A regulates Langerhans cell survival and promotes Treg cell generation upon exposure to ionizing irradiation.
Jeremy G Price, Juliana Idoyaga, Hélène Salmon (…), Saghi Ghaffari, Marylene Leboeuf, Miriam Merad. (2015) Nature Immunology.
-
Treatment with ionizing radiation (IR) can lead to the accumulation of tumor-infiltrating regulatory T cells (Treg cells) and subsequent resistance of tumors to radiotherapy. Here we focused on the contribution of the epidermal mononuclear phagocytes Langerhans cells (LCs) to this phenomenon because of their ability to resist depletion by high-dose IR. We found that LCs resisted apoptosis and rapidly repaired DNA damage after exposure to IR. In particular, we found that the cyclin-dependent kinase inhibitor CDKN1A (p21) was overexpressed in LCs and that Cdkn1a(-/-) LCs underwent apoptosis and accumulated DNA damage following IR treatment. Wild-type LCs upregulated major histocompatibility complex class II molecules, migrated to the draining lymph nodes and induced an increase in Treg cell numbers upon exposure to IR, but Cdkn1a(-/-) LCs did not. Our findings suggest a means for manipulating the resistance of LCs to IR to enhance the response of cutaneous tumors to radiotherapy.
Cancer: A dendritic-cell brake on antitumour immunity.
Miriam Merad, Hélène Salmon. (2015) Nature.
-
Activation of a cellular stress response and the transcription factor XBP1 in dendritic cells has now been shown to limit the cells' ability to stimulate antitumour immune responses in a mouse model of ovarian cancer.
Progress in understanding the pathogenesis of Langerhans cell histiocytosis: back to Histiocytosis X?
Marie-Luise Berres, Miriam Merad, Carl E Allen. (2015) British Journal of Haematology.
-
Langerhans cell histiocytosis (LCH), the most common histiocytic disorder, is characterized by the accumulation of CD1A(+) /CD207(+) mononuclear phagocytes within granulomatous lesions that can affect nearly all organ systems. Historically, LCH has been presumed to arise from transformed or pathologically activated epidermal dendritic cells called Langerhans cells. However, new evidence supports a model in which LCH occurs as a consequence of a misguided differentiation programme of myeloid dendritic cell precursors. Genetic, molecular and functional data implicate activation of the ERK signalling pathway at critical stages in myeloid differentiation as an essential and universal driver of LCH pathology. Based on these findings, we propose that LCH should be re-defined as an inflammatory myeloid neoplasia. Increased understanding of LCH pathogenesis will provide opportunities to optimize and personalize therapy through improved risk-stratification, targeted therapy and assessment of therapy response based on specific molecular features and origin of the pathological myeloid cells.
The non-small cell lung cancer immune contexture. A major determinant of tumor characteristics and patient outcome.
Romain Remark, Christian Becker, Jorge E Gomez (…),Miriam Merad, Sacha Gnjatic. (2015) American Journal of Respiratory and Critical Care Medicine.
-
Solid tumors, beyond mere accumulation of cancer cells, form a complex ecosystem consisting of normal epithelial cells, fibroblasts, blood and lymphatic vessels, structural components, and infiltrating hematopoietic cells including myeloid and lymphoid elements that impact tumor growth, tumor spreading, and clinical outcome. The composition of the immune microenvironment is diverse, including various populations of T cells, B cells, dendritic cells, natural killer cells, myeloid-derived suppressor cells, neutrophils, or macrophages. The immune contexture describes the density, location, and organization of these immune cells within solid tumors. In lung cancer, which is the deadliest type of cancer, and particularly in non-small cell lung cancer, its most prevalent form, reports have described some of the interactions between the tumor and the host. These data, in addition to articles on various types of tumors, provide a greater understanding of the tumor-host microenvironment interaction and stimulate the development of prognostic and predictive biomarkers, the identification of novel target antigens for therapeutic intervention, and the implementation of tools for long-term management of patients with cancer.